Abstract
More than 25% of acute myeloid leukemia (AML) patients have an activating mutation of the fetal-liver tyrosine kinase 3 (FLT3) receptor. FLT3 mutations vary, can be multiple, are often unstable length mutations located in the receptor's juxtamembranous domain (internal tandem duplication (ITD) mutations), and represent the strongest single marker for relapse of AML. When developing novel inhibitors of FLT3, it should be taken into careful consideration that there is a high inter- and intra-patient variability in the length and sequence repeated within ITD mutations. Recently, the first FLT3 targeting kinase inhibitor midostaurin was approved for combination therapy in FLT3 mutated AML, and several other kinase inhibitors are at present also in preclinical development. These inhibitors share broad kinase specificity, in several cases co-inhibiting FLT3 with structurally related targets like c-Kit and AXL. These targets may add therapeutic effect as well as adverse effects. Highly specific kinase inhibitors against varying FLT3-ITDs are to our knowledge currently not in clinical development.
We are developing a novel class of highly specific FLT3-ITD targeting kinase inhibitors based on a well-established chemical backbone previously used in well-tolerated anti-viral therapy. These inhibitors, the BiKin2 compounds, are specifically developed to have superior selectivity towards FLT3-ITD over closely related type III tyrosine kinases, minimizing the chances of adverse off-target effects. We are screening the compounds to identify analogues that target a wide variety of FLT3-ITD mutations, and that can be combined with other BiKin2 analogues and/or with other targeted therapies in order to avoid development of acquired resistance during therapy.
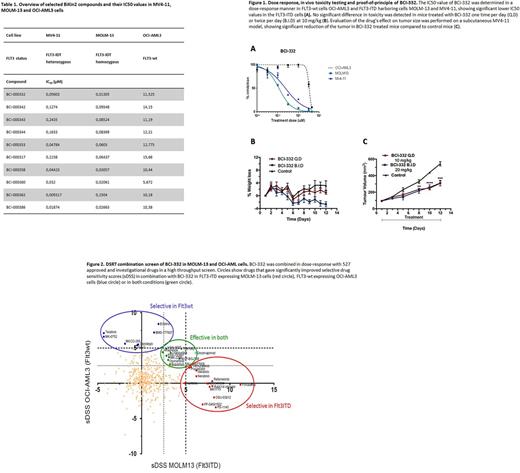
By employing cell lines expressing wild type (wt) FLT3 and ITD mutations of varying lengths we are currently testing more than 40 analogues of the BiKin2 compounds in in vivo and in vitro settings. Among the leukemic cell lines being used are the FLT3-mutated cells MOLM-13 and MV4-11 and the FLT3-wt cells OCI-AML3. Additionally, we are using the BA/F3 cells as a model of FLT3-ITD oncogene addiction. The BA/F3 cells are stably transduced to express FLT3-ITD of different lengths cloned from an AML patient at diagnosis and relapse, in addition to specific point mutations affecting the kinase domain. These screens give us valuable information on how the BiKin2 compounds inhibit and affect ITDs of varying length and sequences. Thus far these novel compounds have shown promising results in ITD mutated cell lines, as shown in Table 1 and Figure 1A.
We have piloted an in vivo toxicity test (MTD) and proof-of-principle-experiments with the first compound showing promising selectivity for FLT3-ITD, BCI-332. We found no dose-limiting toxicity at doses up to 10 mg/kg (Figure 1B), and the drug also showed significant inhibition of tumor growth in a subcutaneous model of FLT3-ITD expressing MV4-11 (ITD/null) cells (Figure 1C). The FLT3 selectivity of BCI-332 at 1 µM over 250 kinases in vitro is superior with 90% versus below 20% inhibition.
In the clinic, patients often develop resistance to FLT3 inhibitors due to secondary mutations within the mutated gene as well as selection of resistant leukemic clones. Therefore, we used a high throughput in vitro drug sensitivity and resistance test (DSRT) approach to evaluate the efficacy of the model compound BCI-332 in a combination screen together with 527 clinically approved and investigational drugs. Drug combinations were tested in both FLT3-ITD expressing (MOLM-13 wt/ITD) and FLT3-wt expressing (OCI-AML3 wt/wt) cells, and we identified several potential drug candidates that showed additive effect in combination with BCI-332 in both conditions (Figure 2). Potential combination candidates need to be further validated in more AML cell lines and in primary cells from AML patients with defined ITD mutational status.
In summary, we employed a novel approach to develop FLT3-ITD targeting drugs by screening inhibitors for selectivity against multiple different length ITD mutations, in an attempt to identify FLT3-ITD specific inhibitors with minimal off-target inhibition. Ultimately, our goal is to identify one or several drug candidate(s) to be tested in a clinical trial setting, in combination or in sequences combined with other targeted therapies.
Amiable: BCI Pharma: Employment. Popa: Kinn Therapeutics AS: Employment. Guillon: BCI Pharma: Employment. Heckman: Novartis: Research Funding; Orion Pharma: Research Funding; Pfizer: Research Funding; Celgene: Research Funding; IMI2 project HARMONY: Research Funding. McCormack: Kinn Therapeutics AS: Other: Co-ownership. Surleraux: BCI Pharma: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal